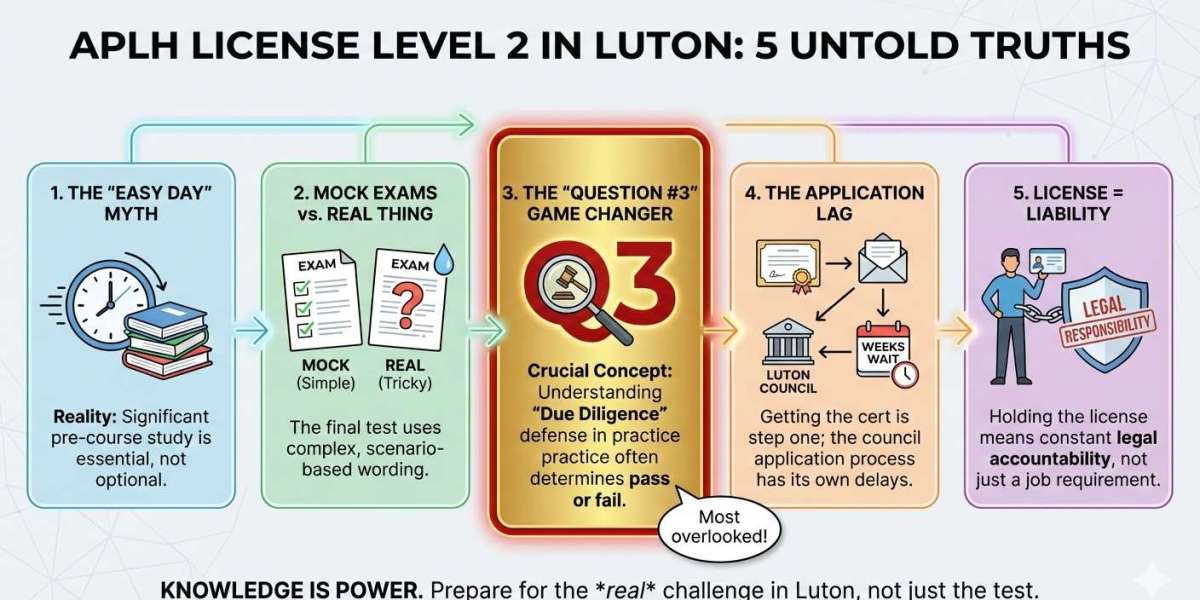
In chemical research and industrial applications, high-purity reagents are essential for reliable results. Lithium Hydroxide is a versatile compound used extensively in laboratories, especially in synthesis processes. ACS reagent lithium hydroxide monohydrate provides the purity and stability required for precise chemical reactions.
Lithium hydroxide monohydrate (LiOH·H₂O) is an inorganic compound containing lithium, oxygen, hydrogen, and one water molecule in its crystalline structure. This monohydrate form enhances stability and handling, making it ideal for chemical synthesis and laboratory studies.
With its strong alkaline properties and high solubility in water, Lithium Hydroxide is widely used in neutralization, pH adjustment, and the preparation of lithium salts. Its predictable behavior and high purity make it an indispensable reagent in both research and industrial chemistry.
Chemical Composition & Properties
ACS reagent lithium hydroxide monohydrate is a white crystalline or granular powder with the molecular formula LiOH·H₂O and a molar mass of 41.96 g/mol.
Key Properties:
Appearance: White crystals
Solubility: Highly soluble in water
pH: Strongly alkaline
Hygroscopic: Can absorb moisture and CO₂ from the air
Proper storage in airtight containers is critical to prevent moisture absorption and the formation of lithium carbonate, which could reduce its reactivity.
ACS Reagent Purity
The ACS reagent grade indicates high purity and strict adherence to chemical standards. This ensures minimal contamination, reliable performance, and consistent results in chemical synthesis.
Benefits of ACS reagent grade lithium hydroxide monohydrate include:
Consistent chemical reactivity
Low impurity levels
Reliable behavior in synthesis and analytical applications
High-purity reagents are critical in research and industrial applications where precision and reproducibility are required.
Laboratory Applications
Lithium Hydroxide is widely used as a strong base in laboratories. Key applications include:
pH Control: Neutralizes acids and prepares alkaline buffers
Synthesis of Lithium Compounds: Forms lithium salts for research and industrial use
CO₂ Absorption Studies: Reacts to form lithium carbonate for environmental simulations
Analytical Chemistry: Prepares standard solutions and calibration reagents
The ACS reagent grade ensures that chemical reactions are consistent, accurate, and reproducible.
Industrial and Research Uses
ACS reagent lithium hydroxide monohydrate is also important in industrial and advanced research applications:
Materials Science: Used in the synthesis of lithium-based ceramics and specialty glass
Energy Research: Supports studies of lithium-ion battery materials
Environmental Chemistry: Applied in controlled CO₂ absorption and air purification experiments
Its predictable chemical properties make it suitable for sensitive experiments and industrial processes.
Safety Guidelines
Lithium hydroxide monohydrate is strongly alkaline and requires careful handling.
Protective Measures:
Wear gloves, goggles, and lab coats
Work in a well-ventilated area or fume hood
Avoid skin, eye contact, and inhalation
First Aid:
Skin: Rinse with water
Eyes: Flush with water for 15+ minutes
Inhalation: Move to fresh air
Proper safety protocols ensure safe use in laboratories and industrial settings.
Storage
To maintain ACS reagent purity:
Store in airtight, moisture-resistant containers
Keep in a cool, dry place
Avoid repeated exposure to air to prevent formation of lithium carbonate
Correct storage ensures the reagent’s stability and reliability.
Disposal
Lithium hydroxide is highly alkaline and should not be released directly into drains.
Disposal Tips:
Neutralize with a suitable acid before disposal
Follow local chemical waste regulations
Avoid contact with soil or water sources
Responsible disposal protects the environment and laboratory personnel.
Advantages of ACS Reagent Lithium Hydroxide
Using ACS reagent lithium hydroxide monohydrate offers several benefits:
Reliable and consistent chemical reactions
High purity for accurate results
Safe handling with proper protocols
Versatility in laboratory and industrial applications
Its high solubility, strong alkalinity, and purity make it an essential reagent for chemical synthesis and research applications.
Conclusion
ACS reagent lithium hydroxide monohydrate is a high-purity, versatile compound ideal for chemical synthesis, laboratory studies, and industrial applications. As a trusted Lithium Hydroxide reagent, it provides consistent reactivity, strong alkaline properties, and reliable performance.
Following proper handling, storage, and disposal guidelines ensures safe and effective use, making this reagent indispensable for modern research, chemical synthesis, and industrial processes.